
When the central metal atom adds electrons from Lewis base/ ligands, it adds a maximum of : Thus, the central atom is capable of accommodating electrons in its s, p, and d orbitals. In a compound, the central atom makes bonds by adding electrons to its orbitals.

The majority of these compounds are solid at normal temperature, while others, such as methylcyclopentadienyl manganese tricarbonyl, are liquid.The distinguishing characteristic of organometallic compounds is the metal-carbon bond that is highly covalent.Organometallic compounds are also called organyl compounds and are prefixed with “organo” before their names. The metal link can include alkali and transition metals, as well as metalloids.
SIGMA BOND EXAMPLE PLUS
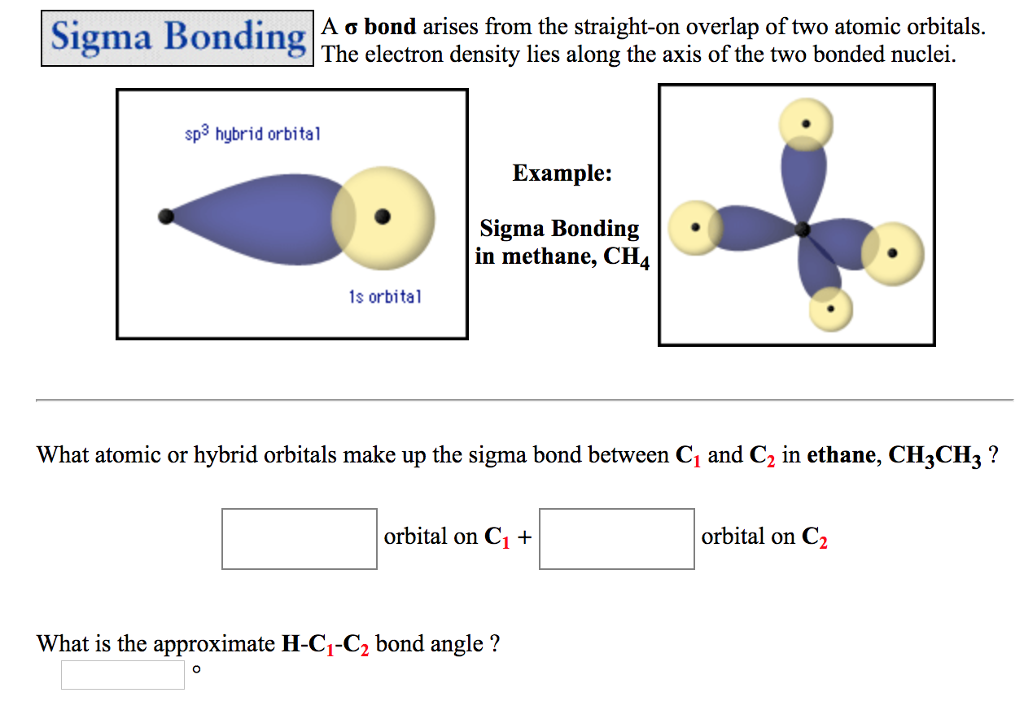
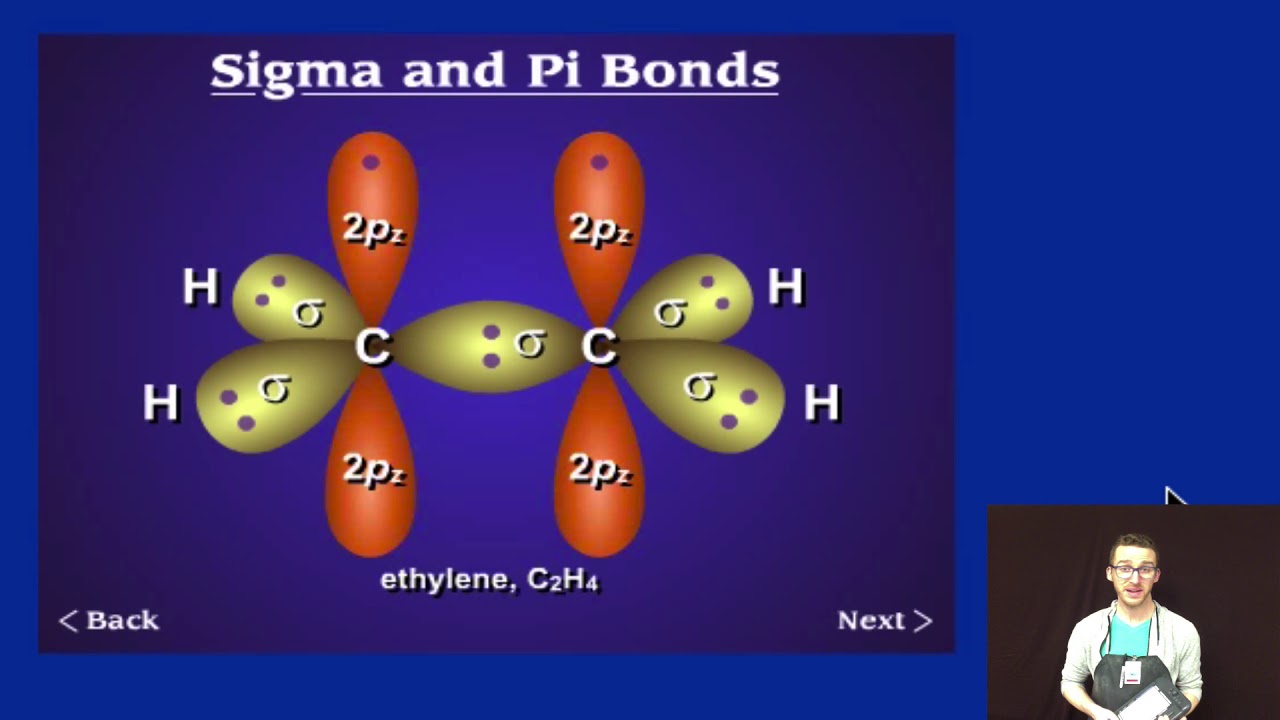
This rule fails further when considering other shapes - toroidal fullerenes will obey the rule that the number of sigma bonds in a molecule is exactly the number of atoms plus the number of rings, as will nanotubes - which, when drawn flat as if looking through one from the end, will have a face in the middle, corresponding to the far end of the nanotube, which is not a ring, and a face corresponding to the outside.Organometallic compounds are compounds having at least one chemical link between a carbon atom from an organic molecule and a metal. Ordinarily, one extra face is assigned to the space not inside any ring, but when Buckminsterfullerene is drawn flat without any crossings, one of the rings makes up the outer pentagon the inside of that ring is the outside of the graph. This is because the sigma rule is a special case of the Euler characteristic, where each ring is considered a face, each sigma bond is an edge, and each atom is a vertex. This rule fails in the case of molecules which, when drawn flat on paper, have a different number of rings than the molecule actually has - for example, Buckminsterfullerene, C 60, which has 32 rings, 60 atoms, and 90 sigma bonds, one for each pair of bonded atoms however, 60 + 32 - 1 = 91, not 90. In this case there are 16 C−C sigma bonds and 10 C−H bonds. The anthracene molecule, C 14H 10, has three rings so that the rule gives the number of sigma bonds as 24 + 3 − 1 = 26. Molecules with rings have additional sigma bonds, such as benzene rings, which have 6 C−C sigma bonds within the ring for 6 carbon atoms. There is no more than 1 sigma bond between any two atoms. This rule is a special-case application of the Euler characteristic of the graph which represents the molecule.Ī molecule with no rings can be represented as a tree with a number of bonds equal to the number of atoms minus one (as in dihydrogen, H 2, with only one sigma bond, or ammonia, NH 3, with 3 sigma bonds). According to the sigma bond rule, the number of sigma bonds in a molecule is equivalent to the number of atoms plus the number of rings minus one. Organic molecules are often cyclic compounds containing one or more rings, such as benzene, and are often made up of many sigma bonds along with pi bonds. These sigma bonds can be supplemented with other bonding interactions, such as π-back donation, as in the case of W(CO) 3( PCy 3) 2(H 2), and even δ-bonds, as in the case of chromium(II) acetate. Transition metal complexes that feature multiple bonds, such as the dihydrogen complex, have sigma bonds between the multiple bonded atoms. For example, propane is described as consisting of ten sigma bonds, one each for the two C−C bonds and one each for the eight C−H bonds. The concept of sigma bonding is extended to describe bonding interactions involving overlap of a single lobe of one orbital with a single lobe of another. Sigma bonds are obtained by head-on overlapping of atomic orbitals.


 0 kommentar(er)
0 kommentar(er)
